Продукция
Продукты для растворения лекарственных средств являются важными инструментами для фармацевтических испытаний, предназначенными для измерения скорости высвобождения активных ингредиентов из твердых лекарственных форм. Эти продукты включают в себя аппараты для растворения, сосуды и аксессуары, обеспечивающие соответствие мировым фармакопейным стандартам. Они обеспечивают точные, воспроизводимые результаты, что имеет решающее значение для разработки лекарств, контроля качества и утверждения регулирующими органами. Идеально подходит для лабораторий, стремящихся к точности и надежности при испытаниях на растворение.
-
Фармацевтическое растворение
-
Трансдермальная диффузия
-
Новый анализатор соединений
-
Автоматизация лабораторий
-
Аналитический прибор
-
Фармацевтические физико-химические свойства

Фармацевтическое растворение
※Compliance with Chinese, U.S. and EU Pharmacopoeia, and other national regulatory standards. ※Complies with 21 CFR Part 11 audit requirements. ※Covers all dissolution test devices mentioned in regulations USP 1-USP 4, as well as related mechanical performance test kits and solvent handling equipment. ※Built-in, easy-to-use operation interface. ※Comprehensive range of dissolution test accessories and rapid customization service.


Трансдермальная диффузия
The USP demands a manual and automatic transdermal diffusion cell system to test semi-solid preparations which this system meets. The testing system enables research and development of IVRT and IVPT methods for specific drug products including topical preparations and patches to examine their safety levels and effectiveness. The RT800 Franz Diffusion Cell performs precise transdermal drug tests through automated sampling in alignment with USP chapter <1724>. The shorter design structure of this apparatus minimizes specimen residue thus resulting in better measurement accuracy and reliability. The device implements audit trail functions that meet the demanding data integrity regulations that pharmaceutical scientists require.

Новый анализатор соединений
The drug analyzer incorporates an algorithm-based software program for accurate detection of drug molecules as it helps investigate the pharmacokinetic characteristics of prescription medicines. The analysis tool conducts a prescription analysis of drug preparations by creating simulated human bodies in test environments that support drug research and development with reference opinions.

Автоматизация лабораторий
An advanced laboratory solution for pharmaceutical applications named the RT900 Multi Batch Automatic Dissolution System operates in the industry. This system runs dissolution tests for 10 batches while delivering precise media dosages and completes a complete cleaning sequence for dissolution cups. Its complete automation configuration leads to improved accuracy and cuts down human involvement to advance research development speed. The system has embedded advanced technological capabilities that enable smooth data acquisition as well as processing and reporting functions. Standardized operations together with the intelligent workflow of the RT900 system deliver dependable experimental results of superior quality.
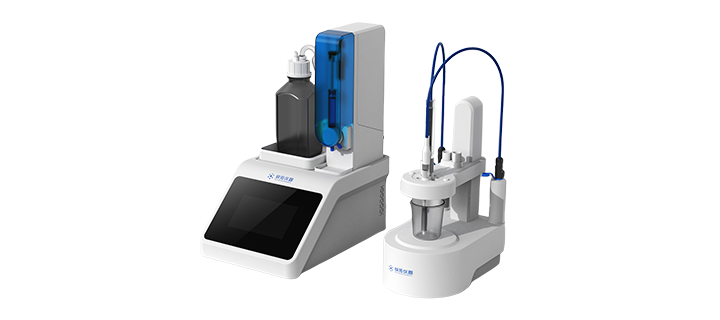
Аналитический прибор
The Potentiometric Titrator serves as a precise analytical instrument that brings accuracy to its titration analytic capabilities. The device comes with a small design that enables rapid setup in addition to straightforward laboratory workflow entry. The instrument features an interface designed for easy use and a screen that enhances user experience. Secure results documentation depends on an efficient data storage system that also provides reliable and safe operations. Laboratory efficiency increases through its designed efficiency which adds precise automated titration functions.

Фармацевтические физико-химические свойства
The RT150 Tablet Disintegration Tester serves together with the RT100 Tablet Breaking Force Analyzer as a pharmaceutical quality control device. Compliance standards are met by the RT150 through its adjustable hanging basket and self-governing dual-group framework. A built-in lighting system allows users to operate the instrument under improved conditions and achieve stable performance. This instrument measures tablet hardness accurately through its built-in pressure detection module. Step-up analysis and quick deployment are possible because of their compact nature along with the independent sample collection box that enables efficient laboratory analysis.


Safe
Economical
Reliable
-
Usp Specifications
-
High-Volume Production with Fast Delivery
-
High Precision Accuracy
-
Engineering support
-
24/7 Sales support
Have a Question ? We Are Here to Help
-
Q:
How do Raytor's products support the pharmaceutical industry?
-
Our products are designed to assist in drug formulation and quality control, offering solutions for oral, transdermal, and subcutaneous dosage forms. We focus on providing accurate data for drug release, absorption, and overall product stability.
-
Q:
Are Raytor's instruments compliant with international standards?
-
Yes, Raytor's instruments are designed and manufactured to comply with international standards. The company adheres to various quality and safety regulations to ensure that their products meet the required performance and safety criteria across different markets.
-
Q:
What kind of after-sales support does Raytor offer?
-
Raytor provides a comprehensive range of after-sales support to ensure customer satisfaction and the proper functioning of their instruments.
-
Q:
Can Raytor customize instruments for specific laboratory needs?
-
Yes, Raytor offers customization of instruments to meet specific laboratory needs. They work closely with customers to tailor features, functionality, and configurations based on unique requirements, ensuring optimal performance for specialized applications. This may include modifications to design, software, or technical specifications. Customization options are available for both medical and industrial instruments.
-
Q:
What makes Raytor's laboratory instruments unique compared to others?
-
Raytor's laboratory instruments stand out due to their combination of precision, reliability, and advanced technology.
-
Q:
What types of laboratory scientific instruments does Raytor provide?
-
Raytor provides a wide range of laboratory scientific instruments, including spectrometers, microscopes, centrifuges, pH meters, incubators, autoclaves, and chromatography equipment. Their products cater to various fields such as life sciences, pharmaceuticals, chemistry, and material testing, offering high precision and reliability.





